Limit 2 per order*
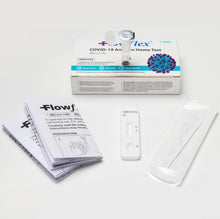
Flowflex COVID-19 Home Test, FDA authorized Antigen test that requires only 1 test, results in 15 minutes, Nasal Swab.
Description
-
NO PRESCRIPTION REQUIRED – AUTHORIZED under FDA EUA (emergency use authorization) for home use
- DETECTS ACTIVE COVID-19 INFECTION – Antigens indicate active disease, find out if you have COVID-19 by performing a painless nasal swab
-
TEST ONCE – unlike other tests, no need to retest a couple of days after the 1st test, unless new symptoms appear!
-
NO SYMPTOMS Needed – The Flowflex COVID-19 Home Test has been authorized to test patients with or without COVID-19 symptoms
-
QUICK – Get Results in 15 minutes. Straightforward and easy to read
- ACCURATE RESULTS – that let you enjoy family time, while worrying less about COVID-19.
- Clinical performance showed 93% sensitivity and 100% specificity
- TEST CHILDREN AGES 2 and up, who are too young for vaccinations. Now everyone can enjoy the family for the upcoming holidays.
Please follow directions provided on product packaging.
Warnings:
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVDs for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.